Our latest blog series is designed to guide peptide chemists towards a greener, more sustainable laboratory. Each of our blog entries (or sermons, if you will) will delve into one principle. If you missed our previous “sermon,” there is always a chance at redemption: all will eventually be posted on our blog page, found here.
Second Commandment: Atom Economy
This principle states that “Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product.”1
Just as Berkeley W. Cue, Jr., Ph.D.was able to distill the First Principle down to its essence,1 so too did the developer of the Second Principle of green chemistry.3 It can be simply stated as the “atom economy” of a reaction. Atom economy, which Barry Trost developed, asks the question, “what atoms of the reactants are incorporated into the final desired product(s) and what atoms are wasted?”2
Going back to 1949, J. Leggett Bailey was involved with the question of atom economy, more likely to shorten reaction times, easier preparation, and a higher yield of the target peptide than advance the idea of “green chemistry”.3,4
Eventually, the formal calculation of atom economy was represented as:1
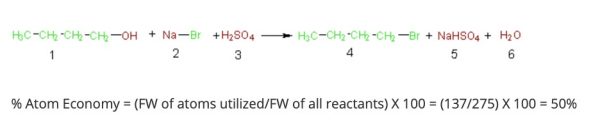
Michael Cann, Ph.D., Professor of Chemistry, at the University of Scranton, explains this, “as the percent atom economy is simply the formula weight of the desired product(s) (compound 4, 137 g/mol) divided by the sum of the formula weights of all the reactants (275 g/mol), which gives 50% in this case. Simply put, even if our percent yield is 100%, only half the mass of the reactants atoms are incorporated in the desired product while the other half is wasted in unwanted by-products.” 2 He succinctly lays out the dilemma as being that: “chemists must not only strive to achieve maximum percent yield, but also design syntheses that maximize the incorporation of the atoms of the reactants into the desired product.” 1
However, as peptide chemists well know, the formula is even more complicated when substituting those reactants for other materials such as solvents and separating agents during a synthesis. These issues will be addressed in the forthcoming “Commandments” article.
New synthetic method development that cuts costs, saves time and reduces waste is one approach to improving atom economy. By exploring and implementing efficient, new synthetic technologies such as those GAP Peptides offers, new and sustainable processes that reduce waste can improve profit and yield. Solvents and separating agents are addressed in forthcoming “Commandments” or principles also.
Many other valuable resources listed below can assist you with adhering to the “Second Commandment.”
References
- https://www.acs.org/content/acs/en/greenchemistry/principles/12-principles-of-green-chemistry.html
- B.M. Trost, Science, 254, 1471 (1991).
- J.L. Bailey, Nature, 164, 889 (1949). https://doi.org/10.1038/164889a0
- Z. Wang (Editor) Comprehensive Organic Name Reactions and Reagents, 3 Volume Set, ISBN: 978-0-471-70450-8 (2009).
Resources
- 1998 PGCCA Winner: Professor Barry M. Trost of Stanford University, “The Development of the Concept of Atom Economy.” https://www.epa.gov/greenchemistry/presidential-green-chemistry-challenge-1998-academic-award-trost
- http://academic.scranton.edu/faculty/cannm1/organicmodule.html
- https://www.acs.org/content/dam/acsorg/greenchemistry/redesign/principles/the-12-principles-of-green-chemistry-pocket-guide.pdf
- https://www.acs.org/content/acs/en/greenchemistry/principles/12-principles-of-green-chemistry.html
- https://www.acs.org/content/acs/en/greenchemistry.html
- https://www.epa.gov/greenchemistry/basics-green-chemistry
- https://www.epa.gov/greenchemistry
- https://pubs.acs.org/doi/10.1021/jo4020722

