The Commandments of Green Chemistry
Our latest blog series is designed to guide peptide chemists towards a greener, more sustainable laboratory. Each of our blog entries (or sermons, if you will) will delve into one principle. If you missed a previous one, they can be found here.
Eighth Commandment: Reduce Derivatives
The full explanation of this principle is: “Unnecessary derivatization (use of blocking groups, protection/deprotection, temporary modification of physical/chemical processes) should be minimized or avoided, if possible because such steps require additional reagents and can generate waste.1” As has been the case with the previous entries, a closer reading may be in order. There are some key, qualifying adverbs. This time we would point out “unnecessary” and “temporary”, along with the phrase, “should be minimized or avoided, if possible” are all crucial to adhere to the intent without stifling innovation. There are numerous reagents that are “green” as well and designed to be less impactful environmentally. Also, as with other metrics, in green chemistry, reagents rest on a continuum and they should be evaluated accordingly rather than rejected outright. The main considerations are derivatizations or modifications. Are they temporary and/or necessary?
As Peter J. Dunn, Green Chemistry Lead at Pfizer clarifies in this principle’s entry on the ACS website: “A great example of the use of enzymes to avoid protecting groups and clean up processes is the industrial synthesis of semi-synthetic antibiotics such as ampicillin and amoxicillin.1”
Many antibiotics used today are derived from peptides. While it is better known as an “antibiotic” rather than a peptide, it is nonetheless a compelling and apt illustration.2,3 Dunn continues demonstrating the principle by describing that, “In the first industrial synthesis Penicillin G (R=H) is first protected as its silyl ester [R = Si(Me)3] then reacted with phosphorus pentachloride at -40o C to form the chlorimidate (1) subsequent hydrolysis gives the desired 6-APA from which semi-synthetic penicillins are manufactured.
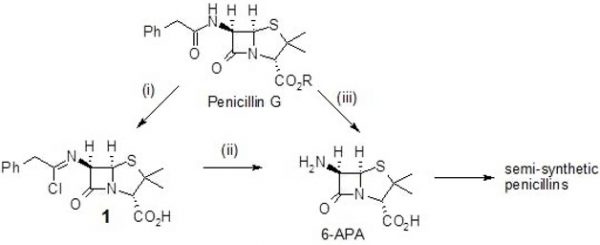
Industrial synthesis of penicillin
(i) TMSCl then PCl5, PhNMe2, CH2Cl2, -40oC (ii) n-BuOH, -40o C, then H2O, 0oC (iii) Pen-acylase, water
This synthesis has been largely replaced by a newer enzymatic process using pen-acylase. This synthesis occurs in water at just above room temperature. The new synthesis has many advantages from a green perspective one of which is that the silyl protecting group is not required.
More than 10,000 metric tons of 6-aminopenicillanic acid is made every year and much of it by the greener enzymatic process, so this is a fantastic example of Green Chemistry making a real difference.1”
References
- https://www.acs.org/content/acs/en/greenchemistry/principles/12-principles-of-green-chemistry.html
- https://pubmed.ncbi.nlm.nih.gov/28469278/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3347563/

