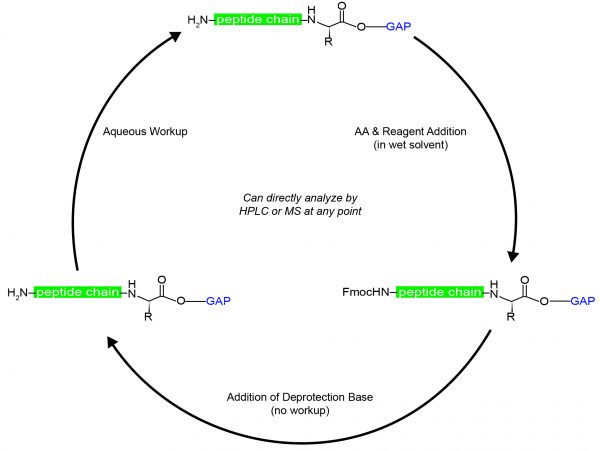
How Group Assisted Purification (GAP) Peptide Synthesis Works
GAP Peptide Synthesis (GAP-PS) was invented by Dr. Cole W. Seifert and Dr. Guigen Li, a distinguished Paul Whitfield Horn Professor at Texas Tech University. GAP-PS is based on concepts derived from GAP chemistry, which has been more broadly applied to organic synthesis.
GAP-PS achieves solubility control over the growing peptide by the attachment of an intelligently designed small-molecule protecting group to a substrate of interest. During GAP-PS, the anchor group attaches to the C-terminus of the first amino acid, from which coupling and deprotection reactions are run in solution phase using standard Fmoc chemistry. Rather than using excess amounts of organic solvent for washing and intermediate purification, GAP-PS washes the organic layer with water to remove synthesis byproducts. While standard chemistry does not require precipitation or filtration, the GAP anchor can facilitate selective precipitation of the peptide at any time, allowing removal of organic byproducts and enabling a wide variety of possible chemistries.
GAP-PS is highly amenable to Fmoc chemistry but also handles Cbz and Boc protection strategies. Because our GAP protecting group functions just like any other C-terminal protecting group, all coupling chemistries are amenable including carbodiimide, uronium, ammonium, cyanuric acid, fluoride, and Oxyma based products.