Our latest blog series is designed to guide peptide chemists towards a greener, more sustainable laboratory. Paul Anastas and John Warner laid the principles out in their book, Green Chemistry: Theory and Practice.1 As the pioneer of green chemistry, Yale professor Paul Anastas, who subsequently was awarded the prestigious Royal Society of Chemistry Award, set out to define the specific tenets required to achieve more sustainable chemistry processes. Each of our blog entries (or sermons, if you will) will delve into one principle.

First Commandment: Prevent Waste
This principle states that “Design chemical syntheses to prevent waste. Leave no waste to treat or clean up.” Or as Berkeley W. Cue, Jr., Ph.D., of BWC Pharma Consulting, LLC, has interpreted: “It is better to prevent waste than to treat or clean up waste after it has been created.”2 He believes that this principle takes precedence over all others and that the other eleven are ways to achieve the first.
Of course, one critical question beyond all the apparent safety, health, and ecological concerns: why prevent waste? As Michael Kopach from Eli Lilly shared in his presentation, “The Business Case for Green Chemistry” at the TIDES conference last year, the volume of waste generated in traditional peptide synthesis and what that equates to in dollars spent just to deal with it is astounding! Kopach references B.W. Cue’s and Wei Zhang’s book that addressing these issues.3,4 In another article, this one quoting Jan Pawlas, Ph.D., a scientist in global development at the PolyPeptide Group, saying, “The current (traditional solid-phase peptide synthesis) methods can produce any peptide at scale, but have a big impact on the environment. Producing one kilogram of the GLP-1 agonist exenatide generates up to 34 tons of waste and 118 tons of carbon dioxide.” 5 That waste in its original form has a cost, along with a steep price associated with its removal. (See our previous blog on economy)
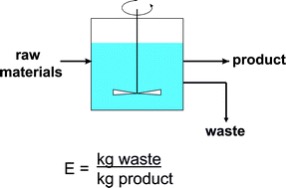
One way to measure the prevention of waste is by a formula called the “E-Factor.” As the creator of the E-Factor, Roger A. Sheldon, a recognized authority of green chemistry, asserts in his paper “The E-Factor: Fifteen Years On,” “We conclude that the E-Factor concept has played a major role in focusing the attention of the chemical industry worldwide, and particularly the pharmaceutical industry, on the problem of waste generation in chemicals manufacture.”6 Simply stated the E-Factor is E=kg waste/kg product.
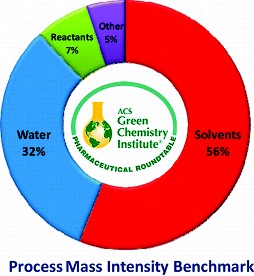
However, a new calculation has gained favor with the American Chemical Society in recent years. The paper, “Using the Right Green Yardstick: Why Process Mass Intensity Is Used in the Pharmaceutical Industry To Drive More Sustainable Processes,” declares in the abstract: “There have been many publications and much discussion about green metrics. While many have been proposed, The American Chemical Society Green Chemistry Institute’s Pharmaceutical Roundtable has chosen Process Mass Intensity (PMI) as the key, high-level metric for evaluating and benchmarking progress towards more sustainable manufacturing.”7,8 For an even more detailed history of the creation E-Factor, its evolution, and the need for additional metrics such as PMI, Roger Sheldon again revisited the topic ten years on in his ongoing series, “The E-Factor 25 Years On: The Rise of Green Chemistry and Sustainability.”9 This entry details some of the advantages and drawbacks of both metrics. For those wanting more details of PMI, you can find a link to the ACS’s PMI Tool here.
Many other valuable resources listed below can assist you with adhering to this “First Commandment.”
References
- P.T. Anastas, J.C. Warner, Green Chemistry: Theory and Practice, Oxford University Press (1998).
- https://www.acs.org/content/acs/en/greenchemistry/principles/12-principles-of-green-chemistry.html
- M. Kopach, The Business Case for Green Chemistry (presentation) (2020).
- W. Zhang, B.W. Cue, Green Techniques for Organic Synthesis and Medicinal Chemistry, John Wiley & Sons (2018).
- https://www.biospace.com/article/tides-polypeptide-group-outlines-cost-effective-green-methods-for-peptide-synthesis/
- R.A. Sheldon, Green Chem., 9, 1273 (2007).
- C. Jimenez-Gonzalez, C.S. Ponder, Q.B. Broxterman, and J.B. Manley, Org. Process Res. Dev., 15 (4), 912 (2011).
- D. J. C. Constable, A. D. Curzons and V. L. Cunningham, Green Chem., 4, 521 (2002).
- R.A. Sheldon, Green Chem., 19, 18 (2017).
Resources

